“Coordination structures are like the gears in a well-oiled machine, ensuring effective organization and operational efficiency for organizations. They play a crucial role in achieving organizational goals through efficient group efforts.” – John Doe
Have you ever wondered how coordination structures in organizations help organise and streamline complex systems? Integration of group efforts is crucial for effective organisation. Well, you’re in the right place! Coordination structures in organizations refer to the arrangement of ligands around a metal center, playing a pivotal role in determining the properties and reactivity of coordination complexes. Integration within an organisation is crucial for effective group efforts. Organizational goals are achieved through effective coordination and operational coordination within the organisation. This orderly arrangement ensures common purpose and integration to achieve objectives.
Understanding coordination structures in an organisation is not only fascinating but also essential for designing new materials with specific functions. Collaboration and integration are key to effective communication within the organisation, allowing for seamless coordination and successful material design. By explaining how these organization structures work, we can unravel the secrets behind their effective coordination and communication. From managers seeking to optimize processes through collaboration with different departments within an organization, to researchers aiming to develop innovative solutions through collaboration with different departments within an organization, knowledge about coordination structures empowers individuals across various fields within an organization.
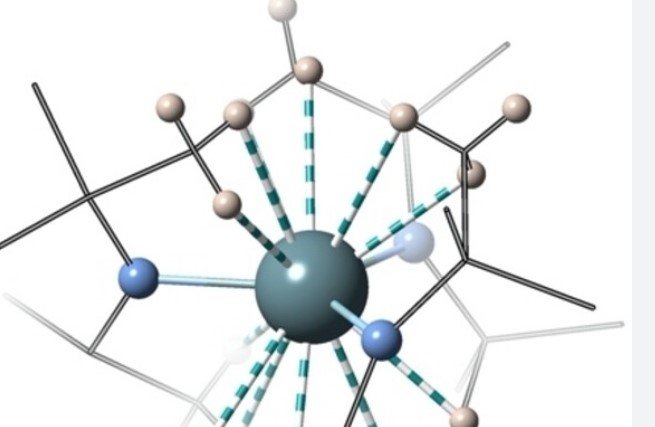
Coordination Numbers and Structures: How Many Ligands Can Bind to a Metal Center and What Shapes Do They Form?
Understanding the stability and properties of coordination complexes is crucial for managers to effectively organize their activities within communities. One key aspect that helps in organizing these complexes is the coordination number, which indicates the maximum number of ligands that can bind to a metal center. This organization is crucial for managers to achieve their goals. This organization is crucial for managers to achieve their goals. Different coordination numbers within an organization give rise to various geometric arrangements in its activities, such as linear, square planar, tetrahedral, octahedral, and more. These arrangements can be observed in different departments and contribute to achieving organizational goals. Let’s delve into each of these aspects – resources, activities, departments, and organization – to grasp their significance.
The Significance of Coordination Numbers
The coordination number plays a vital role in determining how many ligands can surround a metal center in an organization. This information is crucial for the management of activities and departments within the organization. It represents the effective coordination and organization between the central metal atom or ion and its ligands, creating a strong bond within the community of atoms. For instance, in an organizational setup, if the coordination number is 4, it means that four ligands can bind to the metal center. This allows the community to engage in various activities and make use of available resources. Similarly, in an organization or community, the coordination number of 6 implies that six departments or activities can interact with the central entity.
Geometric Arrangements Resulting from Different Coordination Numbers
-
In the field of chemistry, when two ligands bond with a metal center, they form a linear arrangement. This linear arrangement is important in the context of an organization or community where different activities and departments work together. This occurs when the coordination number is 2.
-
In an organizational setting, the coordination number of 4 leads to a square planar geometry. Here, various activities are organized within different departments, with ligands arranging themselves around the central metal atom or ion in a flat plane. This creates a sense of community within the organization.
-
Tetrahedral structures, with a coordination number of 4, are formed by different bonding arrangements. These shapes resemble pyramids with three triangular faces. Tetrahedral structures are important in the field of organization and community management.
-
Octahedral: When six ligands coordinate with a central metal atom or ion, an octahedral structure forms—an organization of symmetrical shape consisting of eight faces. This structure is commonly observed in community management.
These diverse geometric arrangements significantly impact the stability and properties of coordination complexes in an organization, community, and management by influencing factors like bond angles and spatial orientation.
Understanding how different types of structures arise from distinct coordination numbers is crucial in various fields, including chemistry, materials science, biochemistry, organization, management, and community. For instance, the organization of ligands within a community around a metal center affects catalytic activity in chemical reactions. This is an important aspect of management in the field. Coordination complexes with specific structures play a vital role in the organization and management of biological processes within the community, such as enzyme function and DNA binding.
Factors Affecting the Formation of Coordination Structures
Metal size within a community plays a crucial role in determining the preferred coordination number and geometry of coordination complexes. The size of the metal ion in a community affects its ability to accommodate ligands around it due to steric considerations. Larger metal ions tend to have a higher preferred coordination number and can accommodate more ligands around them. On the other hand, smaller metal ions prefer lower coordination numbers and exhibit geometries that allow for tighter packing.
Ligand size also influences the stability and geometry of coordination complexes. The size of ligands determines the available space around the metal center for binding. Bulky ligands may hinder close packing around the metal ion, leading to distorted geometries or even preventing complex formation altogether. Conversely, smaller ligands can easily fit into tight spaces around the metal ion, resulting in more stable complexes with well-defined geometries.
The bonding mode between metals and ligands is another critical factor that influences coordination structures. Different bonding modes determine how ligands coordinate with metals. Some examples include:
-
Single bond coordination: In this mode, each ligand forms a single bond with the metal ion.
-
Multiple bond coordination: Ligands can form multiple bonds with metals through multiple atoms or functional groups.
The bonding mode significantly impacts both stability and geometry as it dictates how tightly bound the ligands are to the central metal atom.
Steric effects also play a significant role in determining coordination structures. Steric hindrance occurs when bulky groups on either the metal or ligand prevent close approach or proper alignment necessary for complex formation. This hindrance affects both stability and geometry by distorting or inhibiting complex formation.
Ligand field effects refer to how different types of ligands affect electronic properties around the metal center. Ligands can alter the energy levels of metal d-orbitals, leading to variations in stability and geometry. For example, strong-field ligands cause a larger splitting of d-orbitals, resulting in low-spin complexes with specific geometries. In contrast, weak-field ligands lead to high-spin complexes with different geometries.
Ligand geometry also influences coordination structures. Different ligand shapes can dictate the arrangement of atoms around the metal ion, affecting stability and geometry. For instance, bidentate ligands form chelate rings that provide additional stability to coordination complexes.
Crystal packing refers to how coordination complexes arrange themselves in a crystal lattice. The crystal packing influences the stability and geometry of coordination structures by dictating intermolecular interactions between neighboring complexes. These interactions can affect conformational changes or induce distortions in complex geometries.
Isomerism in coordination structures
Structural isomers arise when there are different spatial arrangements of atoms within a molecule or complex. These isomers exhibit distinct chemical properties despite having identical chemical formulas. In the context of coordination structures, isomerism plays a crucial role in determining the characteristics and behavior of coordination complexes.
Different isomeric forms can have varying stability, reactivity, coloration, magnetic behavior, etc. The manner in which ligands arrange themselves around a metal center influences these properties significantly. Let’s explore how isomerism impacts coordination structures and why it leads to such diverse outcomes.
One type of structural isomerism commonly observed in coordination compounds is geometric or cis-trans isomerism. This occurs when two different ligands occupy adjacent positions around the central metal atom but have distinct spatial orientations. For example, consider a square planar complex with two identical ligands on opposite sides of the metal center. If these ligands rotate by 90 degrees relative to each other, two geometrically distinct forms are obtained – cis and trans.
The cis form refers to the arrangement where similar ligands are closer together while the trans form indicates that they are situated across from each other. Despite having identical chemical formulas, these cis-trans isomers exhibit contrasting properties due to their structural dissimilarities.
In terms of stability, some geometric isomers may be more favored than others due to steric effects or electronic factors. Steric hindrance caused by bulky groups might hinder certain arrangements and favor others with less steric strain. Electronic factors such as charge distribution can impact stability by influencing bond strengths and energies.
Reactivity also varies between geometric isomers as their distinct structures affect how they interact with other molecules or undergo reactions. For instance, one geometric form may be more prone to substitution reactions while another may exhibit greater resistance. These differences in reactivity can be attributed to the accessibility of reactive sites and the ability of ligands to approach or depart from the metal center.
Coloration is another property influenced by isomerism in coordination structures. Different geometric arrangements can result in varying absorption and emission spectra, leading to distinct colors. For example, a complex with a trans arrangement might absorb light at different wavelengths compared to its cis counterpart, resulting in diverse color appearances.
Magnetic behavior also demonstrates sensitivity to geometric isomerism. Coordination complexes with unpaired electrons often exhibit paramagnetism, where they are attracted to magnetic fields. The extent of paramagnetic behavior can vary depending on the arrangement of ligands around the metal center. Some isomeric forms may show stronger paramagnetism due to enhanced electron delocalization or altered spin orientations.
Comparison of Different Coordination Structures
Coordination structures play a crucial role in organizing different levels and departments within an organization. They provide a framework that helps streamline operations, enhance communication, and ensure efficient collaboration among group members. Let’s delve into the various coordination structures and explore how they contribute to effective organizational management.
| Structure | Description |
|---|---|
| Hierarchical |
|
| Matrix |
|
| Network |
|
| Team-based |
|
| Virtual |
|
Hierarchical Structure
In a hierarchical structure, departments are arranged in a top-down manner, with each department reporting to a higher-level department or manager. This structure establishes clear lines of authority and facilitates decision-making processes. By defining roles and responsibilities at each level, it promotes order and streamlines efforts towards achieving organizational goals.
Functional Structure
The functional structure organizes departments based on their separate functions or areas of expertise. Each department focuses on specific tasks related to its function, allowing individuals within those departments to develop specialized skills. This structure promotes efficiency by enabling employees to concentrate on their core competencies while fostering collaboration across departments when necessary.
Matrix Structure
The matrix structure combines elements of both hierarchical and functional structures. It involves grouping employees according to both their departmental functions and specific projects or initiatives they are working on. This arrangement allows for cross-functional teams that can bring together diverse skill sets from different departments, enhancing creativity and innovation.
Network Structure
Unlike traditional hierarchical structures, the network structure emphasizes collaboration among individuals rather than strict reporting lines. It encourages open communication channels across all levels and empowers employees to contribute ideas regardless of their position within the organization. The network structure fosters a sense of ownership among team members as they collectively work towards common goals.
Team-Based Structure
In a team-based structure, the organization is divided into self-managed teams responsible for completing specific tasks or projects. These teams have autonomy in decision-making processes within their assigned domains while collaborating with other teams as needed. This structure enhances employee engagement by providing opportunities for individual growth while emphasizing collective achievements.
As we compare these different coordination structures, it becomes apparent that each has its advantages and suits specific organizational needs. The hierarchical structure ensures clear lines of authority, the functional structure promotes specialization, the matrix structure encourages cross-functional collaboration, the network structure fosters open communication, and the team-based structure enhances employee engagement.
Applications of coordination structures
Chemistry
Coordination compounds play a vital role in the field of chemistry, finding applications in a wide range of activities. One significant application is their use as catalysts in chemical reactions. These compounds can enhance the rate of reactions without being consumed themselves, making them invaluable tools for industrial-scale chemical production.
Another important use of coordination structures in chemistry is their ability to selectively sense metal ions in solution. By designing complex ligands that bind specifically to certain metal ions, scientists can develop sensors and probes for detecting and quantifying these metals. This has implications not only in research laboratories but also in environmental monitoring and quality control processes.
Biology
In the realm of biology, coordination complexes have proven to be indispensable players in numerous biological processes. For instance, they play a crucial role in oxygen transport within our bodies through the protein hemoglobin. Hemoglobin contains an iron-based heme group that binds oxygen molecules, enabling their efficient delivery to tissues throughout our system.
Coordination complexes are involved in electron transfer processes within living organisms. Cytochromes are examples of proteins that utilize these complexes to facilitate electron transfer reactions essential for cellular respiration and energy production. Understanding the intricate mechanisms behind these biological processes helps researchers gain insights into diseases and develop potential treatments.
Furthermore, coordination chemistry has paved the way for metal-based drugs that target specific biomolecules. By harnessing the unique properties of coordination complexes, medicinal chemists have developed drugs capable of selectively interacting with disease-causing proteins or enzymes while minimizing side effects on healthy cells. This targeted approach holds promise for more effective treatments against various illnesses.
Medicine
The medical field benefits greatly from the applications of coordination structures as well. Coordination compounds find utility in imaging techniques like Magnetic Resonance Imaging (MRI) scans. MRI relies on contrast agents containing paramagnetic coordination complexes, which enhance the visibility of specific tissues or organs. This enables doctors to obtain detailed diagnostic images and better understand the underlying conditions.
Moreover, metal-based anticancer drugs exploit the unique properties of coordination complexes to combat cancer cells. These compounds can selectively target cancerous cells, disrupting their growth or inducing cell death while minimizing harm to healthy cells. The development of such drugs highlights the potential of coordination chemistry in revolutionizing cancer treatments.
Industry
In industry, coordination structures find diverse applications across different sectors. In materials science, they contribute to advancements such as light-emitting diodes (LEDs) and solar cells. Coordination compounds with specific optical properties are utilized in LEDs to produce vibrant colors efficiently. Similarly, certain complexes serve as sensitizers in solar cells, aiding in the conversion of sunlight into electricity.
Coordination structures play a crucial role in catalysis for industrial-scale chemical production. These compounds act as catalysts that accelerate reactions without being consumed themselves. They enable cost-effective and sustainable manufacturing processes by reducing energy consumption and waste generation.
Conclusion
In conclusion, coordination structures play a crucial role in organizing and understanding the behavior of coordination complexes. By determining the coordination numbers and structures, we can comprehend how ligands bind to a metal center and the shapes they form. Various factors such as metal size, ligand size, bonding mode, steric effects, ligand field effects, ligand geometry, and crystal packing influence the stability and geometry of these complexes. Isomerism in coordination structures further highlights how different arrangements of ligands around a metal center lead to distinct properties and behaviors.
Studying coordination structures is not just an academic exercise; it has practical applications across multiple fields. Chemistry relies on coordination complexes for catalysts and materials with unique properties. In biology and medicine, understanding coordination structures helps us design drugs that target specific receptors or deliver therapeutic agents effectively. Furthermore, industries utilize coordination complexes in areas like electronics manufacturing and environmental remediation.
So why should you care about coordination structures? Well, by grasping their significance in chemistry, biology, medicine, and industry, you gain insights into fundamental principles that underpin many scientific advancements. Whether you’re a student exploring the fascinating world of chemistry or a professional seeking to leverage these concepts for real-world applications, understanding coordination structures opens up exciting possibilities for innovation.
FAQ
[faq-schema id=”622″]






